
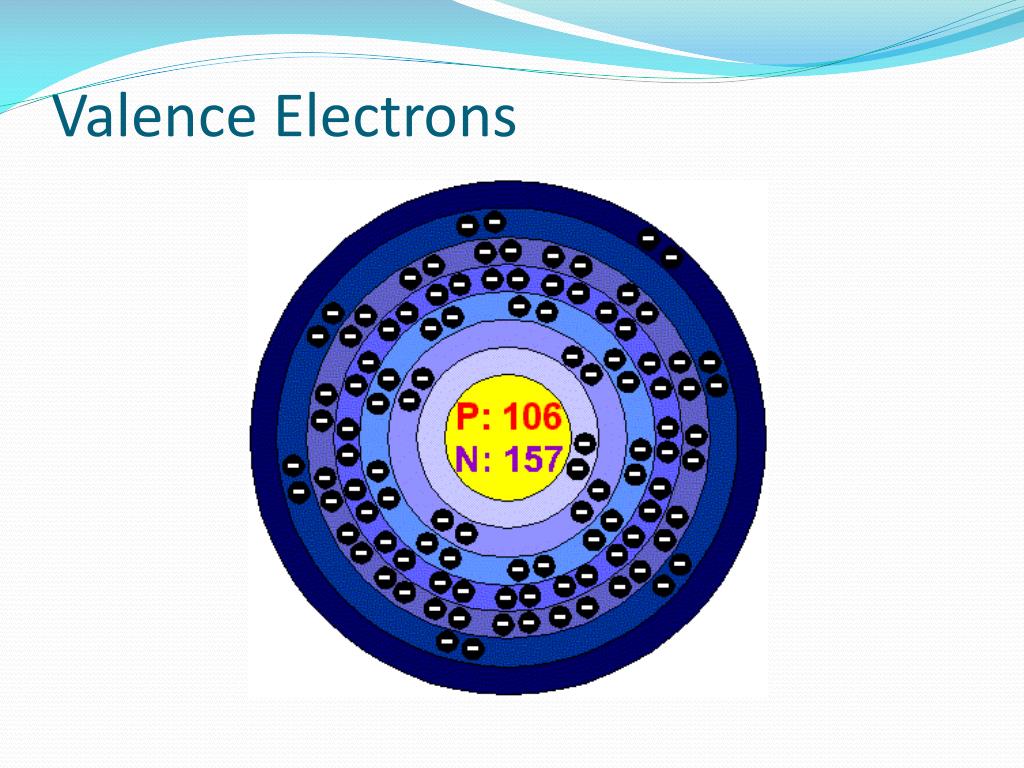
You’d find valence electrons at the outermost shell of an atom. Lewis structure helps us determine valence electrons and how it helps any chemical reaction and predict the type of bond. To see how well you know the information, try the Quiz or Test activity.What is a valence electron? Valence electrons are the electrons in the outermost shell of an atom, while the electrons which are mostly present within the inner circle are known as core electrons. When you need a break, try one of the other activities listed below the flashcards like Matching, Snowman, or Hungry Bug.Īlthough it may feel like you're playing a game, your brain is still making more connections with the information to help you out. If you are logged in to your account, this website will remember which cards you know and don't know so that theyĪre in the same box the next time you log in. LEFT ARROW - move card to the Don't know pile.You can also use your keyboard to move the cards as follows: If you've accidentally put the card in the wrong box, just click on the card to take it out of the box. When you've placed seven or more cards in the Don't know box, click "retry" to try those cards again. If you knew the answer, click the green Know box. Look at the large card and try to recall what is on the other side. Use these flashcards to help memorize information. What are elements such as these described as? Same number of electrons in valence shell.Įlements will give, share, or take electrons to complete a set of electrons. Why are the properties in groups similar? What changes gradually as you move from left to right?Įlements in the same group often have similar _ and _ properties. Where do we find the three types of elements on the periodic table? When did the discoverer of the periodic table discover it? What do electrons rotate the nucleus on or in? Nonmetals: opposite of metals dull, brittle. Metals: luster, good conductors, ductile, malleable. Periodic Law states that the repeating chemical and physical properties of elements change PERIODICALLY with the elements' atomic numbers. Who discovered the number of protons? What year? The strong force keeps particles of _ charges together. The _ force plays an important role in radioactive atomsĮlectromagnetic forces keep _ and _ together. Gravitational, electromagnetic, strong, and weak. In an isotope, the number of _ need to be equal to the number of protons or more.Īn positively charged ion has more or less electrons than protons. This determines an element's identity (two answers).Īre isotopes related to neutrons, electrons, or protons?
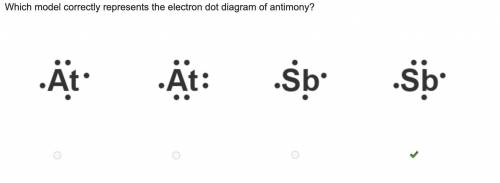
VALENCE ELECTRONS OF ANTIMONY PLUS
This plus number of neutrons equals the atomic number.Īre electrons included in the atomic mass? This person eventually arranged the elements in order of increasing mass. All have eight except for helium, which has two. Helium is in this group, and is an exception to the number of valence electrons. This group of elements have luster, are good conductors, and all have high melting points and densities (except for Mercury). Another element is Germanium, which is used in computer chips. This group includes Ununquadium and are all solids at room temperature. This group includes one metalloid and four metals and has three valence electrons. They have higher densities than alkali-metals. This group includes Beryllium and are all silver in color. This group includes Antimony and has five valence electrons. It is a gas at room temperature and has low density. This group contains a nonmetal that is very reactive.
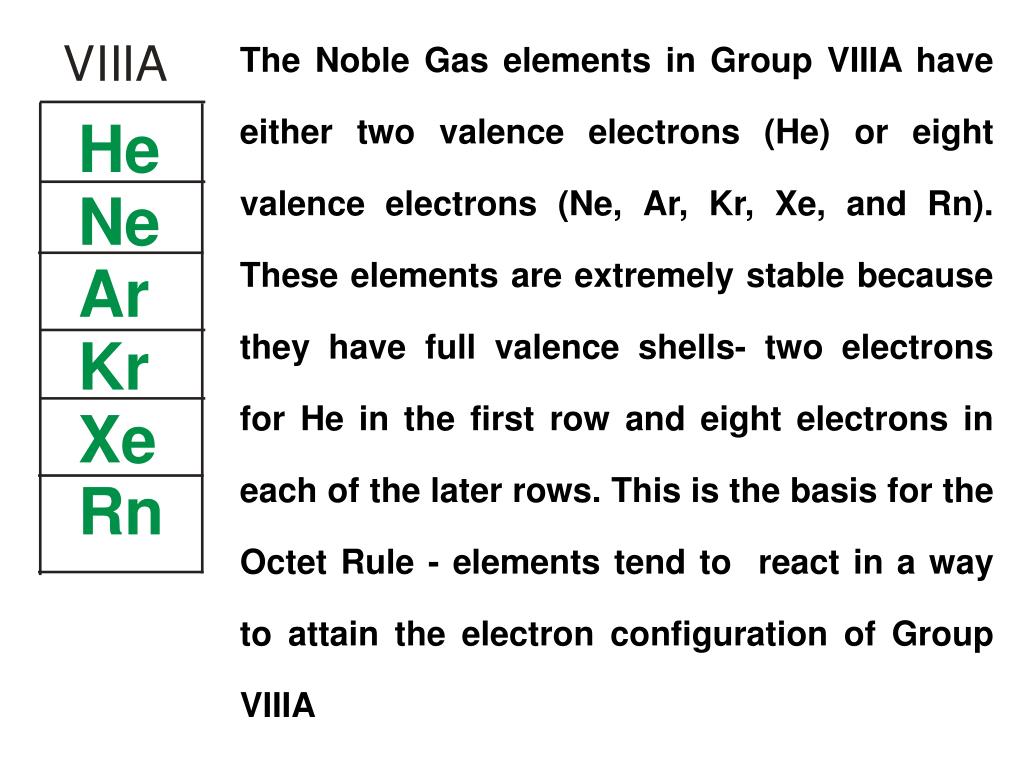
This group is completely nonmetal and are unreactive. What group is completely nonmetal and is VERY reactive? These elements combine with metals to create salts. What group has six valence electrons, three nonmetals, one metalloid, and one metal? One element is also used greatly in chemical industry, and another is very abundant in the air? What group has elements used for fertilizer, reactivity varies, and has five valence electrons? What group has one nonmetal, two metalloids, two metals, and are all solids at room temperature? Elements, such as Tin, are used in cans. What group contains Gallium, has one metalloid and four metals, and are all solid at room temperature? What group includes Calcium, Radium, has higher densities than alkali metals, and some of its elements are used in chalk and airplanes? Lanthanides and Actinides are part of what group? What group of elements has one valence electron in each atom and has low density? Elements Periodic Table of Elements-Families, History, Important Figures Question


 0 kommentar(er)
0 kommentar(er)
